
Other research groups found that the Sec61p channel, a protein-conducting channel on the ER membrane, is involved in GSHĭiffusion from the cytosol to the ER ( Ponsero et al. To transport glutathione through passive transport ( de Crouy-Chanel and Richarme 2001 Bánhegyi et al. The calcium channel ryanodine receptor has been shown in vitro System for GSH and/or GSSG across the ER membrane is available. Of active GSH and/or GSSG transporters on the ER membrane has never been reported. In the oxidative reaction with PDI family proteins and discharge the produced excess GSSG from the ER. Therefore, it is necessary to supply GSH consumed
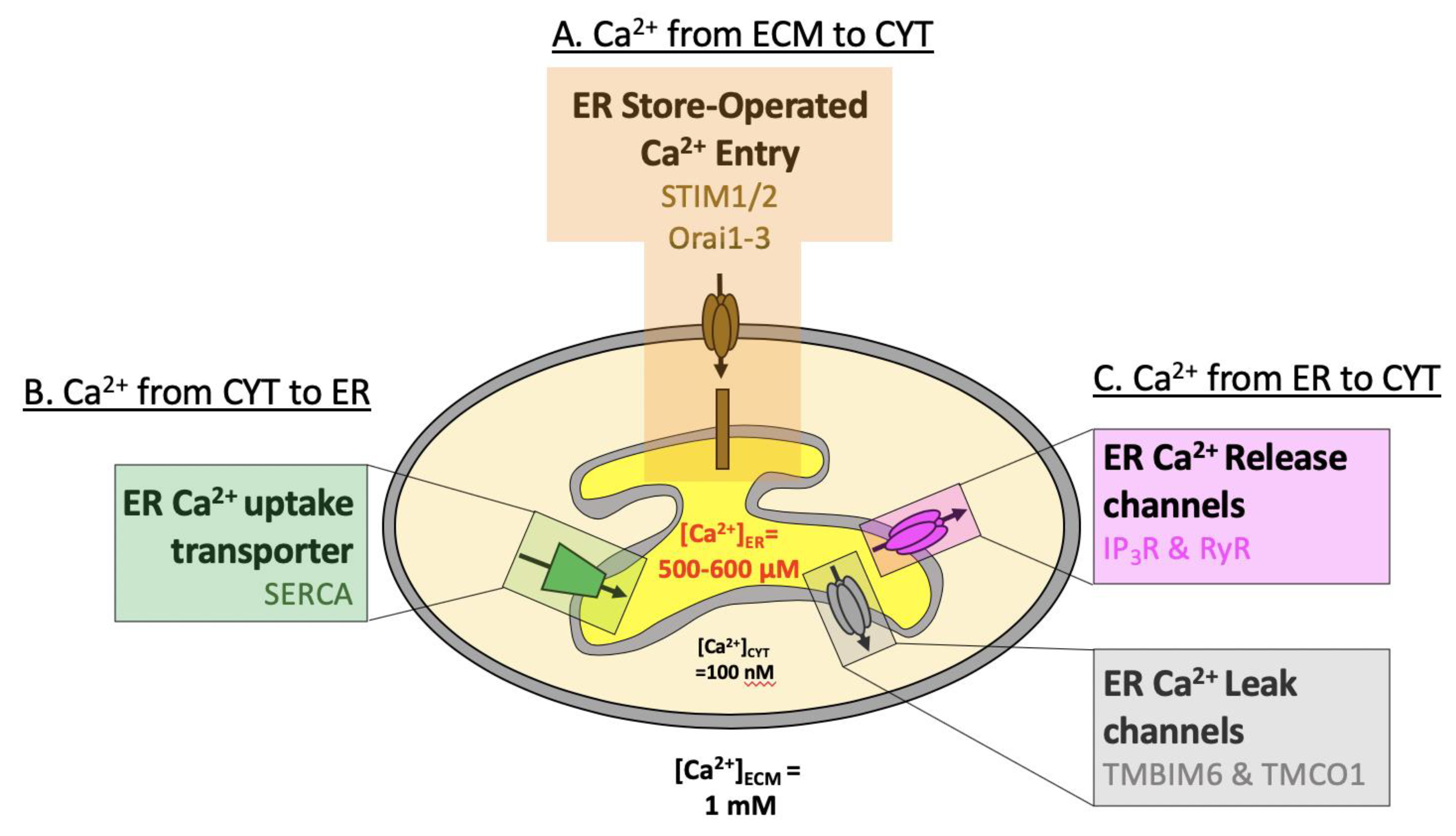
Glutathione synthesis is limited to the cytosol,Īnd the presence of glutathione reductase in the ER has not yet been detected. Later, an electron is finally transferred to molecular oxygen via Ero1). Therefore, GSH reduces PDI family proteins, and this reduction is coupled with oxidation of GSH to GSSG (as will be described In addition, GSH has lower redox potential and higher reactivity than the protein disulfide isomerase (PDI) family proteins,Ī group of oxidoreductases in the ER ( Lappi and Ruddock 2011). In the ER lumen is estimated to be 15 m m, which is higher than that in the whole-cell lysate (7 m m) ( Birk et al. This indicates the existence of a system that transports glutathione to each organelle. Synthesis system is located only in the cytosol ( Delaunay-Moisan et al. In eukaryotic cells, glutathione is present in all organelles, but the glutathione Reduced glutathione (GSH) and oxidized glutathione (GSSG) are in equilibrium and constituteĪ redox environment in each cellular compartment. Glutathione, a tripeptide consisting of glycine, cysteine, and glutamine, is abundant in cells and acts as an electronĭonor in oxidoreductive reactions. Glutathione is one of the major components involved in maintaining the redox environment in both prokaryotic and eukaryoticĬells. Previous Section Next Section REDOX ENVIRONMENT OF THE ER 2009), and metabolic disorders, such as type 2 diabetes ( Back and Kaufman 2012). These failures in ER homeostasis can cause severe diseases, such as neurodegenerative diseases (e.g., Alzheimer'sĭisease) ( Salminen et al. Disruptions in ER homeostasis (generally called ER stress) are triggered by an imbalance among these environmental factors Three major environmental factors, protein quality control, calcium homeostasis, and redox regulation, balance each otherĪnd their cross talk maintains ER homeostasis ( Fig. This oxidative environment is considered advantageous for the disulfide bond formations required for protein folding. 2009).Īnother characteristic feature of the ER environment is that it is oxidative compared with the cytosol ( Hwang et al. 1998 Argon and Simen 1999 Lucero and Kaminer 1999 Michalak et al. Calcium ions in the lumen of the ER are required for the activities of various molecular chaperones, and enzymes in theĮR are also important to maintain protein quality control ( Lebeche et al. Proteins, including calmodulin, in the cytosol ( Berridge et al. Numerous cellular functions, including muscle contraction, cellular motility, and vesicular transport, via calcium-binding Intracellular calcium ions released from the ER are one of the most important signaling molecules in the cytosol and regulate The ER is also a reservoir for intracellular calcium ionsĪnd stores ∼10,000 times more calcium ions than the cytosol ( Meldolesi and Pozzan 1998b Groenendyk et al. 2003), protein quality control is essential to maintain ER homeostasis. One-third of the total proteins produced in whole cells are inserted into the ER ( Ghaemmaghami et al. Therefore, various molecular chaperones and folding enzymes for nascent polypeptides exist in the ER. The endoplasmic reticulum (ER) is the site of folding for newly synthesized secretory and membrane proteins ( Ellgaard and Helenius 2003 Ellgaard and Ruddock 2005).


 0 kommentar(er)
0 kommentar(er)
